How surprising are the ATTRibute-CM results?
BridgeBio announced on December 27th that one of its lead drug candidates had failed to meet its primary endpoint in a phase III trial. As often happens, the company tried to put a positive spin on the press release while the stock plummeted 70%. BridgeBio claims the placebo group declined unusually slowly, and the company pointed to several secondary endpoints that look more encouraging for the drug.
Fascinated as always by clinical trial endpoints, I spent some time digging into the six-minute walk test results.
The ATTRibute-CM trial
The drug candidate in question is acoramidis, a small molecule designed to treat a heart disorder called transthyretin amyloidosis cardiomyopathy (ATTR-CM) by stabilizing the TTR protein. ATTR-CM is characterized by amyloid buildup in the heart, which leads to a stiffening of the heart walls and reduced heart function. There are two types of the disease: hereditary, caused by a mutation in the TTR gene, and wildtype.
Wildtype ATTR-CM is an increasingly common diagnosis. BridgeBio’s pipeline lists the expected patient population as >400,000 in the US and EU, making it one of the most prevalent diseases BridgeBio is going after.
Attribute-CM is a phase III trial to test acoramidis in patients with hereditary or wildtype ATTR-CM. The trial will compare treated patients to a placebo group on two primary endpoints:
- Decline in distance covered on six-minute walk test (6MWT) after 12 months
- Weighted combination of mortality, cardiovascular-related hospitalizations, and decline in six-minute walk after 30 months
The December 27th press release included the results of the first analysis. On average, the acoramidis group had an average decline of 9 meters on the 6MWT, and the placebo group had an average decline of 7 meters. In other words, the drug treatment did not slow the decline and the trial has failed this endpoint.
What do we know about expected decline on the 6MWT?
In their press release, BridgeBio cited two papers that have found a decline of > 40 meters at 12 months. The first was the phase III trial of the only approved therapy for TTR, Pfizer’s Tafamidis (Maurer et al. 2018). The other was a natural history study of 711 patients (Lane et al. 2019).
Let’s look at each of the papers in detail.
Tafamidis phase III trial
The Tafamidis trial placebo arm consisted of 177 patients with a mix of wildtype and mutant ATTR-CM. Figure 4A shows the results on the 6MWT over time.
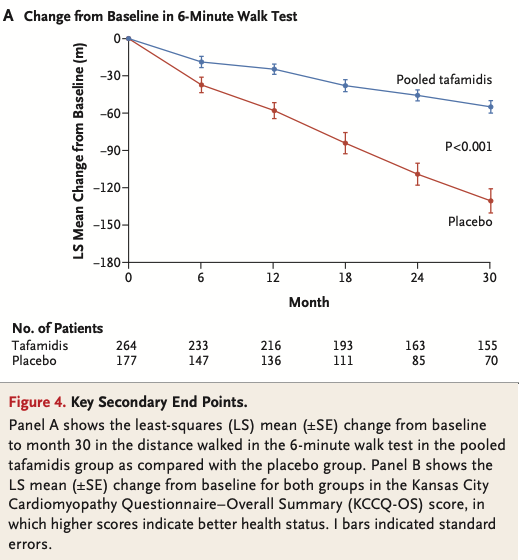
If we extract the 12 month placebo values and calculate the standard deviation, we end up with an average decline of 58 m and an SD of 76.8 m.
Lane et al. natural history study
The Lane natural history study looked at 711 patients in the UK. The larger patient population enabled them to look at results by genotypes. Figure 3 shows the average decline on the 6 MWT, with the error bars giving the 95% confidence interval.
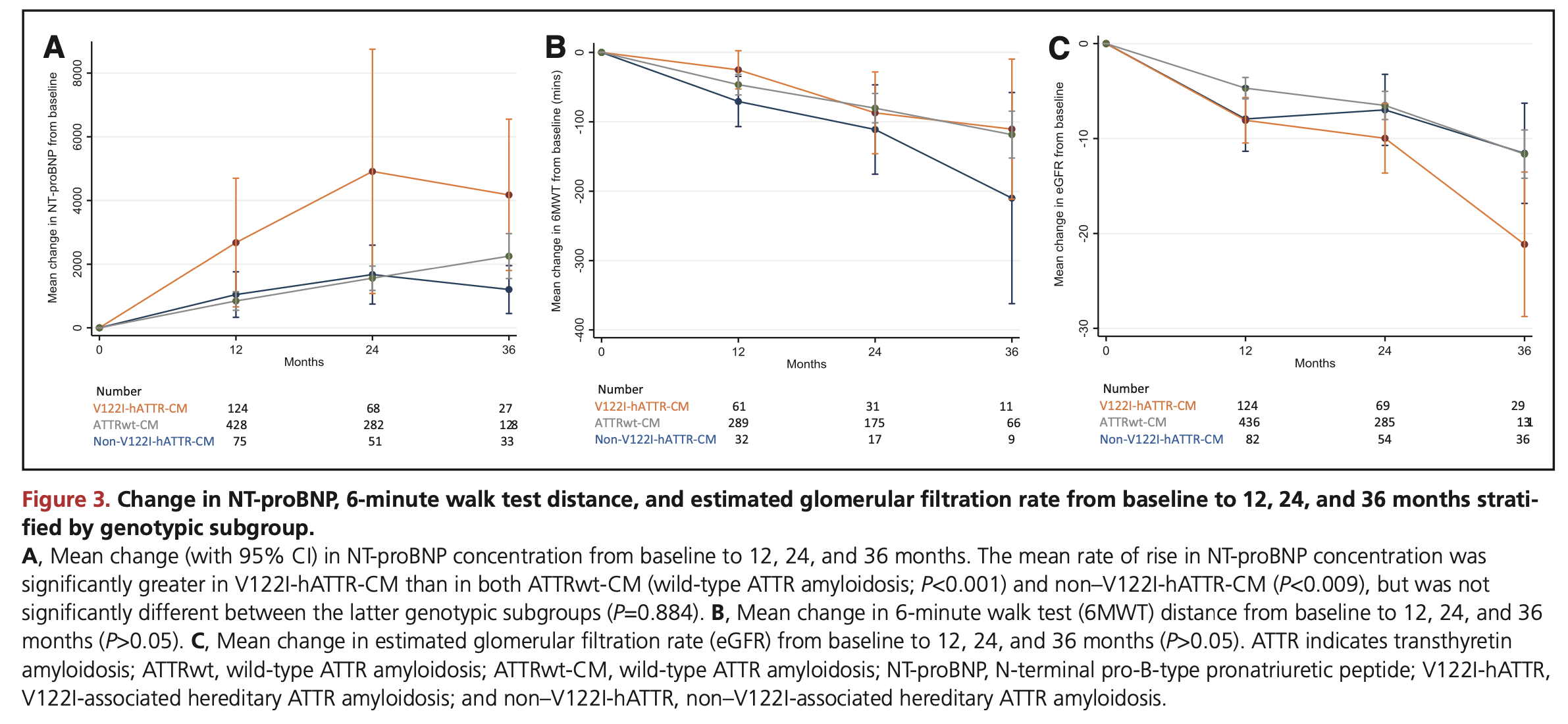
Assuming a normal distribution was used to calculate the CIs1, we can recover the standard deviations at month 12:
- wildtype: mean = -45 m, SD = 127
- V122I variant: mean = -25 m, SD = 111
- other variant: mean = -70 m, SD = 105
The variability is quite a bit larger than in the Tafamidis trial placebo group. As the natural history study ran over a longer time period and had broader inclusion criteria, this isn’t terribly surprising.
Could the 7 meter decline have happened by chance?
The Attribute-CM trial has enrolled 632 participants with a 2:1 randomization between 800 mg of acoramidis and placebo. Using the average decline and variability in the historical data, we can get a sense of what an average decline of 211 placebo patients is likely to look like.
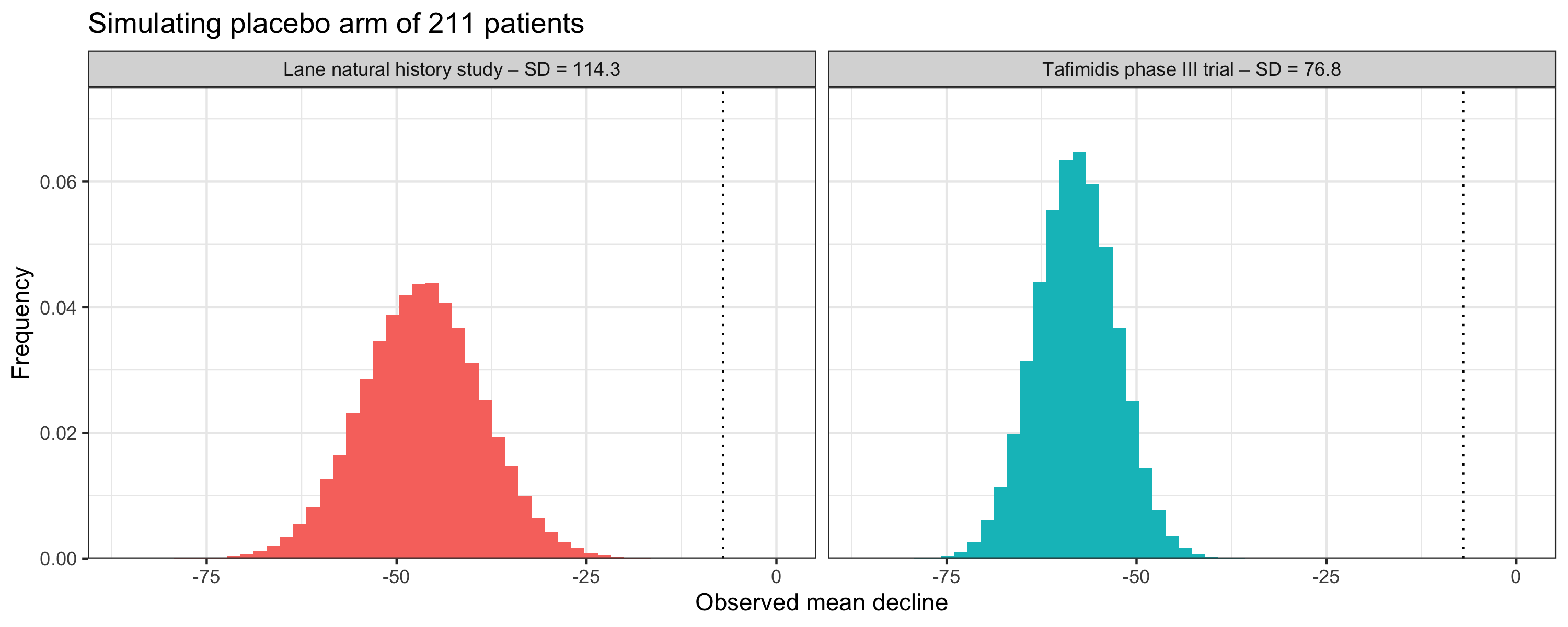
If we (somewhat naively) assume a normal distribution of the 6MWT declines, the observed mean decline of 7 meters is well outside the realm of likely outcomes. This is true regardless of which dataset we use to estimate the expected decline and variability.
Are there systematic differences between the trial and historical data?
If the placebo results aren’t due to random variability, the most likely explanation is that there is a difference in the patient population. Patients in the Attribute-CM trial could be sicker, healthier, or in some other way different, than the patients in the previous studies.
The BridgeBio press release tried to address this possibility head on with the following bullet points:
- Key differences in NYHA class, geographic distribution, and TTR variant status compared to the ATTR-ACT population do not appear to have affected the primary outcome of ATTRibute-CM
- The only participant sub-population the company has reviewed to date that exhibited substantial placebo decline in 6MWT by Month 12 was the variant population. In that population, observed decline in placebo and acoramidis was -40 meters and -2 meters, respectively
They don’t give any information on the baseline characteristics beyond this, so our best indication is the inclusion criteria of the trial. The most relevant ones are:
- 18 to 90 years of age
- wildtype or variant ATTR-CM diagnosed by biopsy or technitium bone scan
- history of heart failure
- New York Heart Association (NYHA) class I-III symptoms
- have completed at least 150 m on 6MWT on two tests
- NT-proBNP levels >= 300 pg/mL
- Left ventricular wall thickness >= 12 mm
The Tafimidis trial used a more limited set of inclusion criteria, only covering diagnosis of ATTR-CM, heart wall thickness, and NYHA class I-III. While the other criteria weren’t explicitly used to filter patients, the parameters that BridgeBio has set are largely consistent with the baseline characteristics reported from the Tafamidis trial.
- Age: median = 74, range = 51 - 89
- 6MWT distance: mean = 353 m, SD = 126
- NT-proBNP levels: median = 3161 pg/ ml, IQR = 1864.4–4825.0
The main difference that stands out is the method of diagnosing ATTR-CM. The Tafamidis trial required a biopsy, while Attribute-CM allowed for diagnosis by technitium bone scan. We have some evidence that patients diagnosed with a bone scan have better prognosis from the Lane natural history study. They found patients diagnosed with wildtype ATTR-CM after 2012, when the bone scan was introduced as an alternative to biopsy, had a median survival of 60.2 months, compared to 46.3 months before 2012. It is not clear whether the differences are driven by stage of diagnosis, as the paper did not perform a multivariate analysis to assess if year of diagnosis is still prognostic after adjusting for the disease stage.
Conclusion
The BridgeBio placebo group results are unusual compared to past data, and unlikely to have happened due to random variability. It is more likely there is some differences in patient characteristics, but at this point we do not know what they could be. When the trial is eventually published, baseline values of age and the 6MWT will give us a better idea of whether there is a difference in patient populations.
Footnotes
This is an overly simplistic assumption, as the population standard deviation is not known.↩︎